6 Protons • 6 Neutrons • 6 Electrons — Understanding Carbon’s Atomic Structure
Last updated: October 29, 2025 • 5–7 min read • Not educational advice
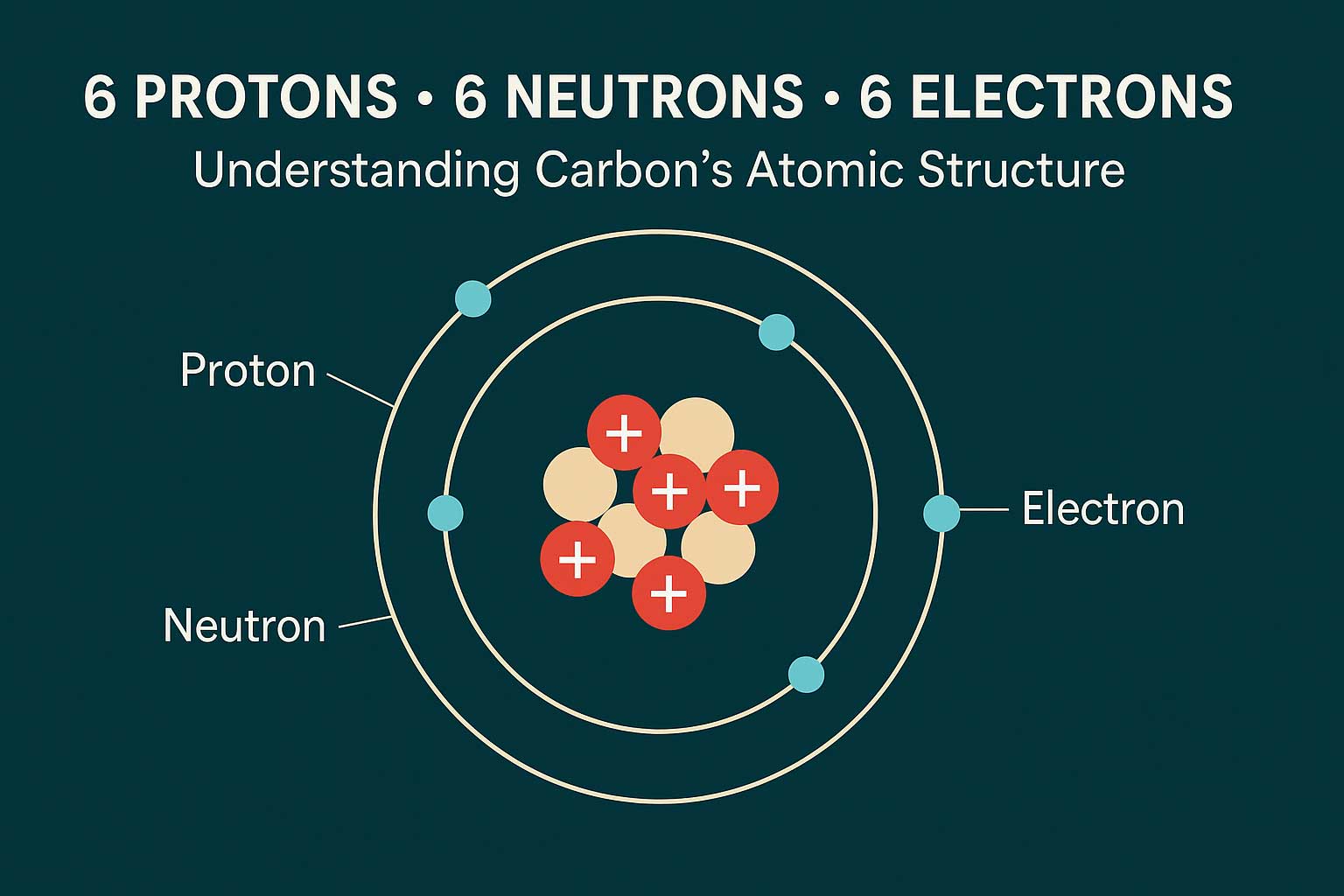
The element carbon (atomic number 6) in its stable isotope form has exactly 6 protons, 6 neutrons, and 6 electrons.
This simple configuration defines its role in materials science, life on Earth, and advanced technologies such as graphene.
This post explores what each subatomic particle does, why that configuration matters, and key takeaways for emerging applications.
What Does “6-6-6” Actually Mean?
When you see “6 protons, 6 neutrons, 6 electrons,” you’re describing the most common stable isotope of carbon — carbon-12.
The atomic number (6) means there are 6 protons in the nucleus, defining the element as carbon.
A neutral carbon atom balances that with 6 electrons orbiting the nucleus, while 6 neutrons contribute mass and stabilize the nucleus.
Why Each Particle Matters
- Protons: Define the element. For carbon, Z = 6 → atomic number 6 means carbon.
- Electrons: Equal to the number of protons in a neutral atom; govern bonding and chemical behavior.
- Neutrons: Add mass and stability, offsetting proton repulsion. Carbon-12 has 6 neutrons (mass = 12 = p + n).
Carbon’s Configuration & Why It Matters
This 6-6-6 configuration matters for several reasons:
- Stability & Abundance: Carbon-12 is stable and forms ~98.9% of all natural carbon.
- Valence & Bonding Versatility: Four valence electrons (2s²2p²) enable up to four covalent bonds — the basis of organic chemistry.
- Materials & Technology Application: This atomic structure supports strong covalent networks in graphene, carbon fiber, and advanced composites.
Common Misconceptions & Clarifications
- “All carbons have 6 neutrons”: Not true — isotopes vary (e.g., ¹³C has 7, ¹⁴C has 8) but all have 6 protons + 6 electrons.
- “Carbon always has atomic mass 12”: Mostly yes for ¹²C, but isotopes shift the mass number (p + n).
- “Electrons don’t matter”: Incorrect — electrons determine bonding, reactivity, and material properties.
Key Takeaways for Materials & Graphene Enthusiasts
In graphene, carbon composites, and nanotubes, this atomic structure explains carbon’s advantages:
a stable nucleus (6 p + 6 n), balanced charge (6 e = 6 p), and versatile bonding.
Claims about “carbon-based” or “graphene-enhanced” materials all trace back to this structure.
FAQ
Why exactly 6 protons and 6 electrons? Can carbon have different numbers?
The 6 protons define carbon’s atomic identity. In a neutral atom, 6 electrons balance the charge.
Carbon can form ions (gaining or losing electrons) or isotopes (changing neutrons),
but neutral carbon-12 always has 6 p, 6 n, 6 e.
Do all isotopes of carbon have 6 protons and 6 electrons?
Yes. All carbon isotopes share 6 protons (atomic number 6) and, when neutral, 6 electrons.
Only the neutron count differs: ¹³C has 7 neutrons, ¹⁴C has 8.
Why is carbon so central to life and materials?
With four valence electrons (2 in inner shell + 4 in outer), carbon forms strong, diverse bonds.
Its stability and abundance make it ideal for biological molecules and high-tech materials like graphene.